
How Element Operates
Element is a manual, portable fire extinguisher. It uses a Potassium salt jet (a unique method among fire extinguishers) that employs the vaporization of the salt in the environment followed by the condensation of its extinguishing substance. Element works by interrupting a fire’s chain of reaction (the “auto-catalyst” of the fire).
Element is composed of stable, solid minerals; it does not contain gas and is not pressurized. The aerosol-like jet is only produced when the charger is struck with its base. The produced jet is free of thrust and is essentially an inert salt that emits gas already present in the atmosphere.
This process allows Element to extinguish all types of fires through saturation, while its slow bio-degradation in the environment, furthers the prevention of subsequent fires.
The Two Reactions
The extinguishing process involves two different reactions: one is physical and the other, chemical.
-

The Physical Reaction
Relates to potassium’s tendency to oxidize rapidly in air. When in contact with air, alkaline salts consume great quantities of oxygen, thus depriving fires of oxygen.
-
The Chemical Reaction
Is created through the stable link between potassium particles and the fire’s combustion particles.
Through the two reactions, a quick oxidation process takes place, immediately transforming the jet from a solid state into a gaseous state freeing the potassium particles. These atoms are able to intercept and interrupt any other free particles produced by the fire’s natural chain reaction combustion process.
Potassium has strong inhibitor qualities due to its weak ionization energies. The extinguishing agent being used is composed of Potassium Ions, organic oxidizer, and plasticizer resin.
When Potassium Ions (KNO3) discharges from the extinguisher it vaporizes in the environment followed by the condensation of its extinguishing substance. When it reacts (inside the body of the extinguisher) it breaks down and the jet that is formed is made up primarily of free radicals of Potassium K+, of Nitrogen N (an inert gas), and water vapor.
The jet that comes out of the unit reacts with the fire. Potassium radicals (K+) capture the Oxygen of the combustion thereby extinguishing it.
Environmentally Friendly
At the end of the extinguishing process the following is discharged to the atmosphere:
-
As a Solid
Particles of Potassium (that have reacted with the Oxygen of the fire) having a size between 3-4 microns. These particles are invisible at sight and heavier than air. They disperse in the atmosphere and tend to deposit on the ground in no appreciable amounts.
-
As a Gas
As Nitrogen; an inert gas already present in the air we breathe at more or less 78%.
-
As Water Vapor
Extremely minimal toxic by-products that are a result of the combustion process.
The Chemical Reaction
The chemical reaction is best illustrated by stages:
-
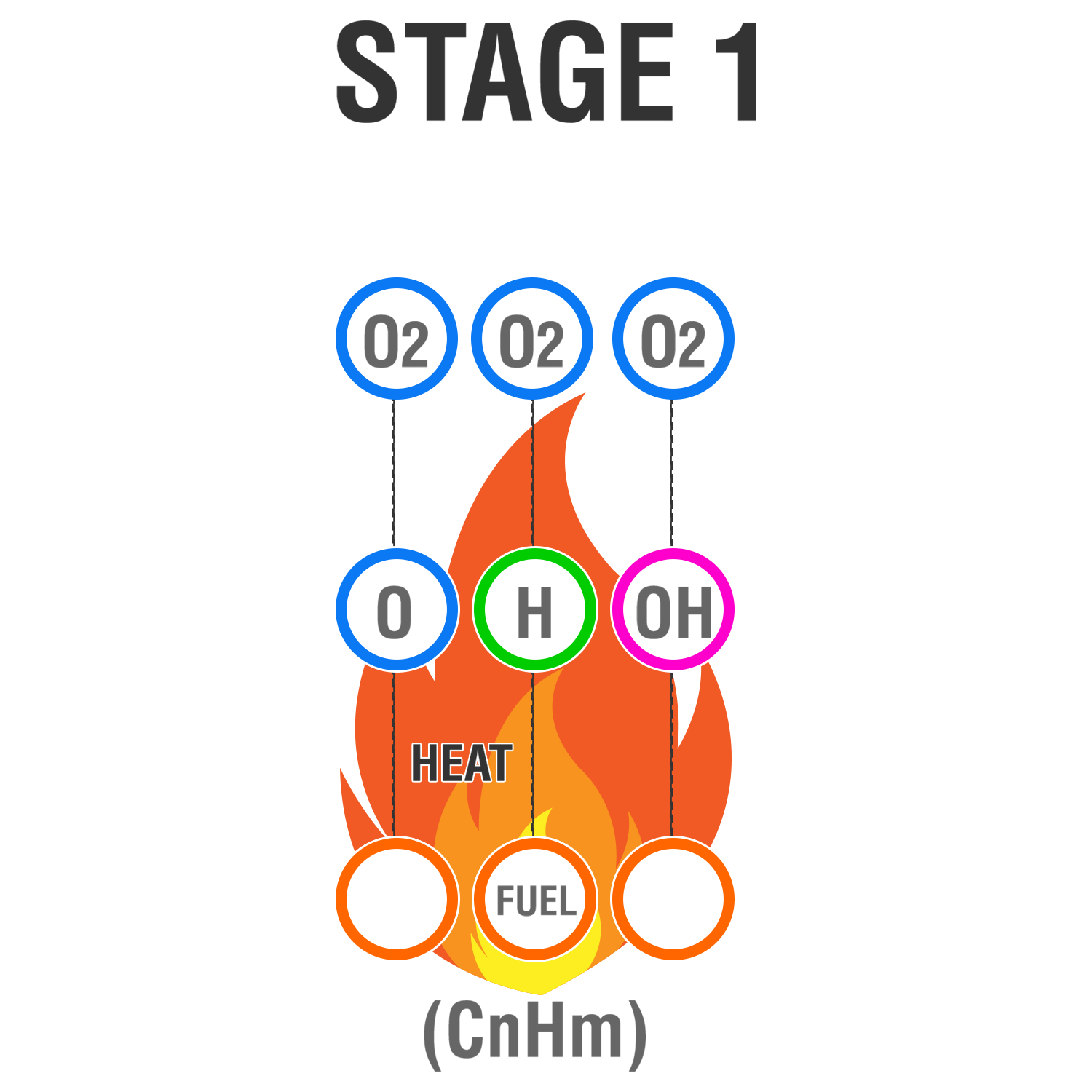
Stage 1
Fire is initiated by the flame chain carriers: O, H and OH
-
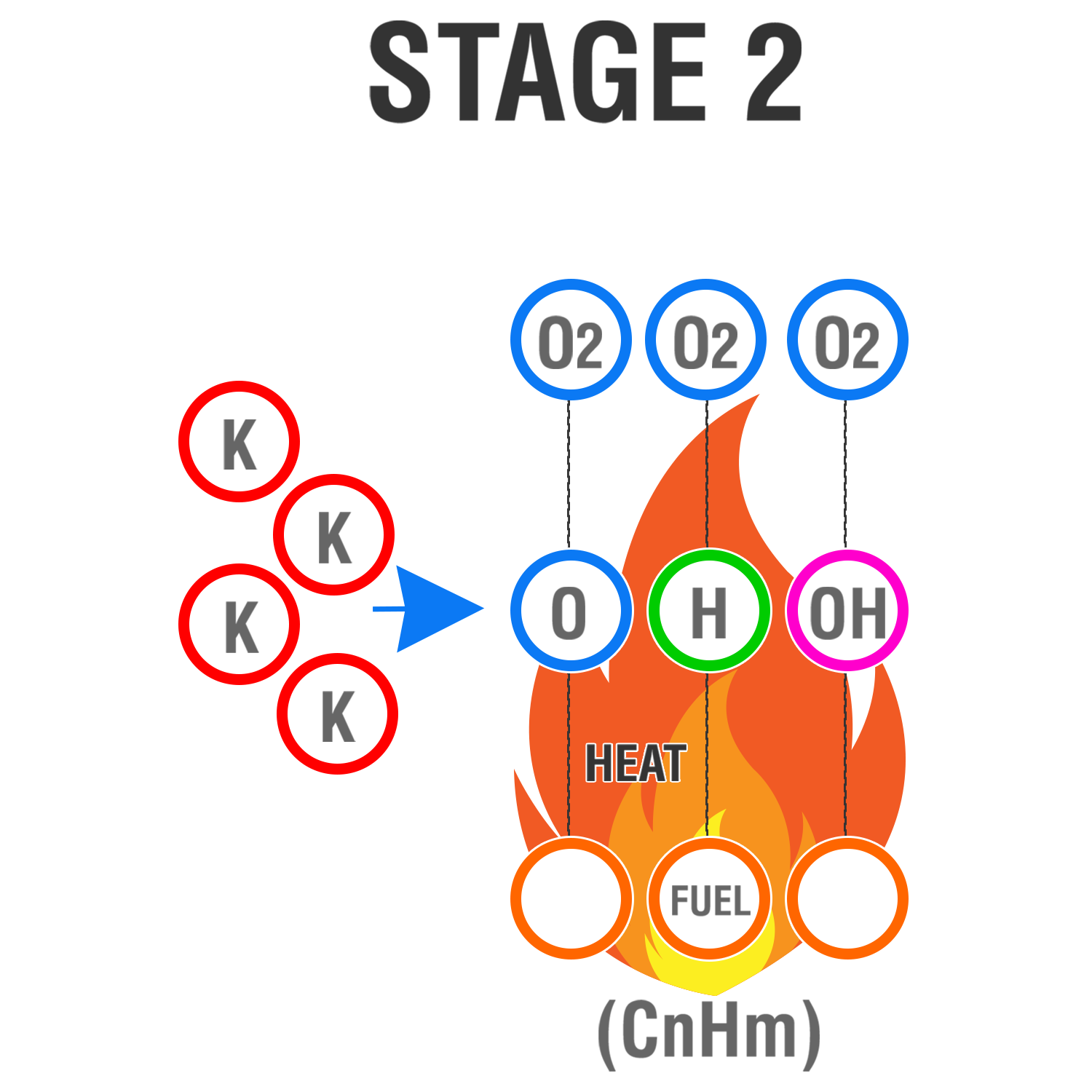
Stage 2
The Element jet introduces Potassium radicals (K) into the flame chain reaction
-
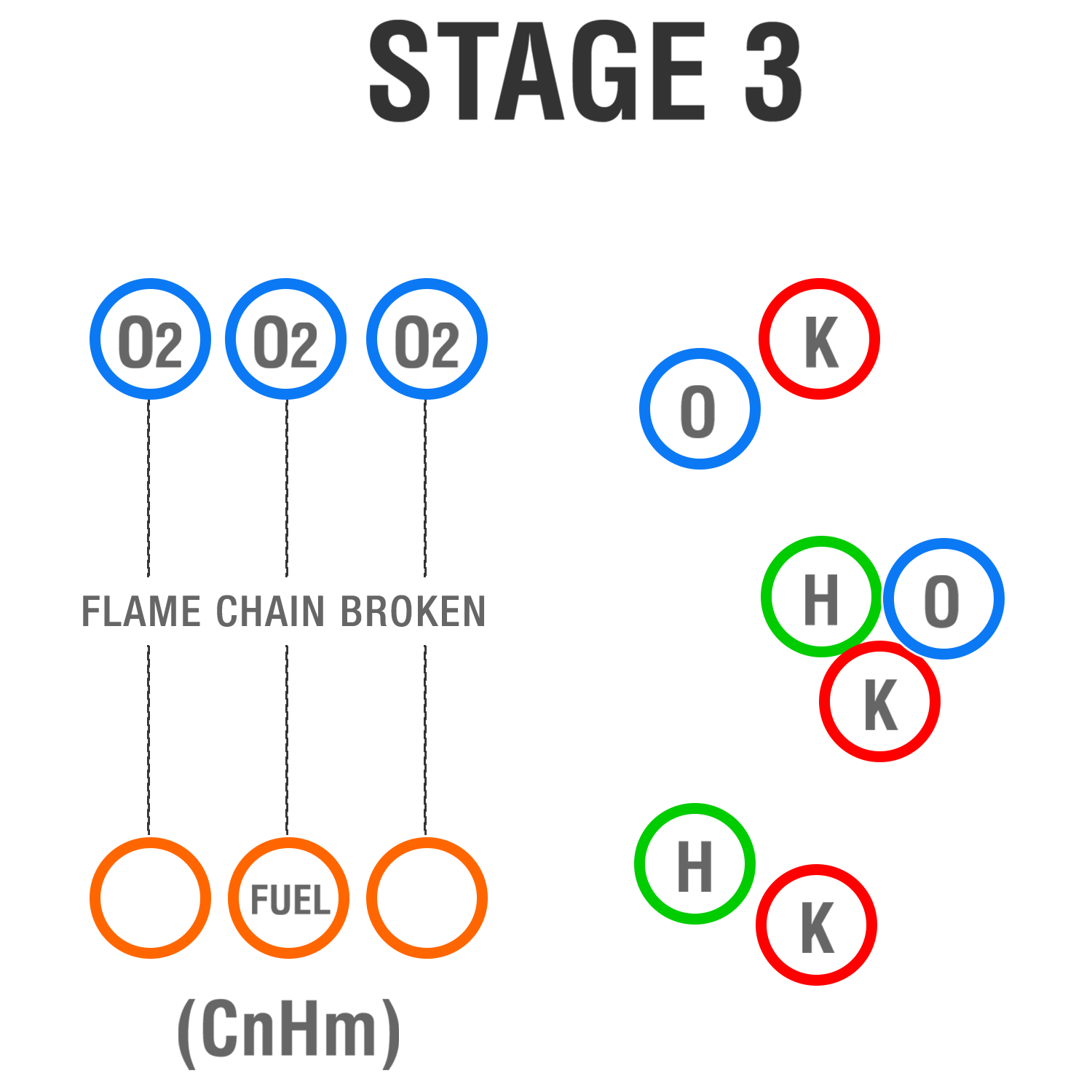
Stage 3
K radicals attach themselves to O, H and OH and remove them from the flame reaction without depleting surrounding Oxygen





